Researchers have identified significant differences between the cells that make up the retinal tissue, which is crucial to human visual perception Scientists from the National Institute of Ophthalmology (NEI) found five subsets of retinal pigment epithelial cells (RPE) - a layer of tissue that nourishes and supports retinal photoreceptors. Using artificial intelligence, the researchers analyzed RPE images at single-cell resolution to create a reference "map" to locate each subgroup in the eye. A report on the study was published in the [proceedings of the National Academy of Sciences] on May 6( https://www.pnas.org/doi/full/10.1073/pnas.2117553119 ) 》Come on.
"These results provide an unprecedented framework for understanding different RPE cell subsets and their vulnerability to retinal diseases and developing targeted therapies to treat them," said Michael F. Chiang, MD, director of Nei at the National Institutes of health
"These findings will help us develop more precise cell and gene therapies for specific degenerative eye diseases," said Dr. Kapil Bharti, the study's lead investigator
Vision begins when light hits rod and cone photoreceptors on the retina behind the eye. Once triggered, photoreceptors transmit signals through a complex network of other retinal neurons that converge at the optic nerve before entering other brain regions. RPE is a monolayer cell located deep in a cell below the photoreceptor.
Age and disease may cause metabolic changes in RPE cells, resulting in the degradation of photoreceptors. The effects of these RPE changes on vision vary greatly depending on the severity and location of RPE cells in the retina. For example, tardive retinal degeneration mainly affects the peripheral retina, thus affecting the peripheral vision. Age-related macular degeneration is the main cause of visual loss. It mainly damages RPE cells in the macula, which are very important for central vision.
Bharti and colleagues tried to determine whether there are different RPE subsets, which may explain the universality of retinal disease phenotypes.
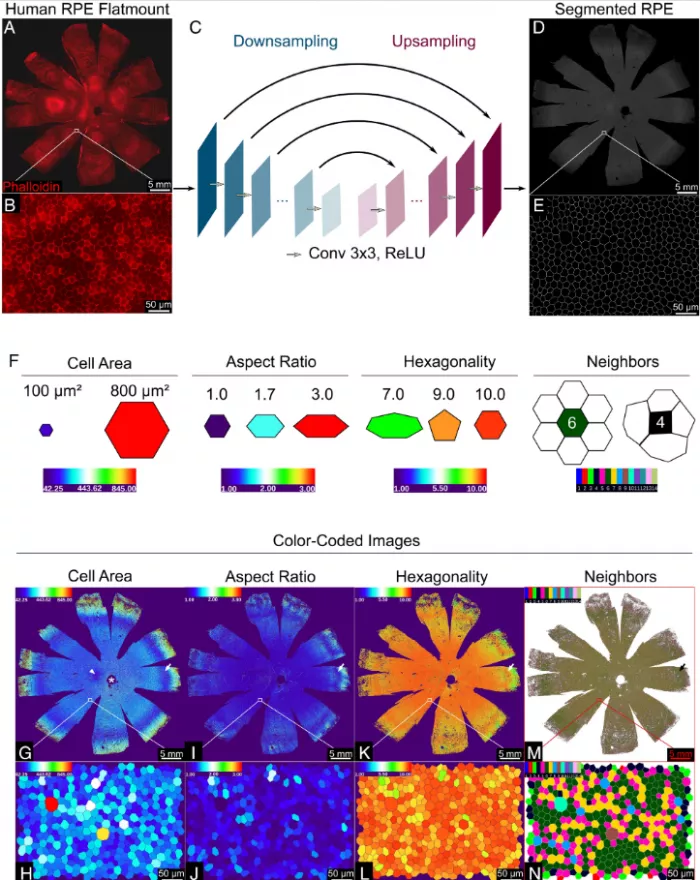
The team used artificial intelligence (AI) to analyze the morphology of RPE cells, that is, the external shape and size of each cell. They trained computers using fluorescent labeled RPE images to analyze entire human RPE monolayers from nine cadaveric donors with no significant history of eye disease.
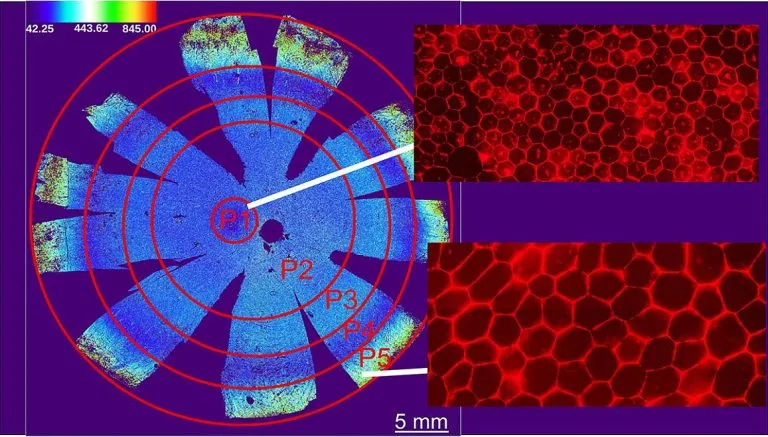
The morphological characteristics of each RPE cell were calculated - an average of about 2.8 million cells per donor; A total of 47.6 million cells were analyzed. The algorithm evaluates the area, aspect ratio (width and height), hexagon and the number of adjacent cells of each cell. Previous studies have shown that the function of RPE is related to the tightness of cell junction; The more crowded it is, the more it can show the health of cells.
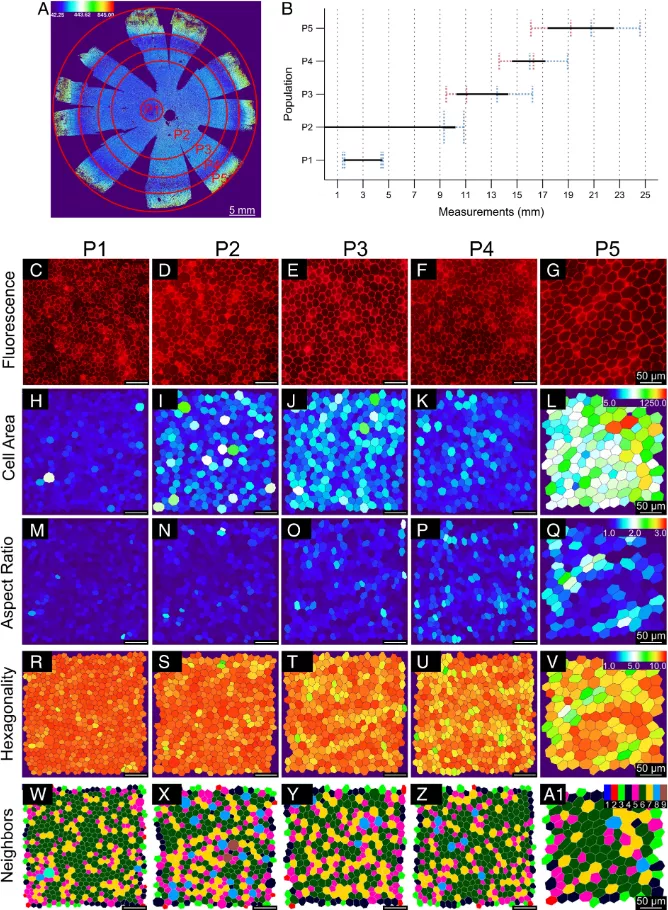
Based on morphological measurements, they identified five unique RPE cell subsets, classified as p1-p5, and grouped them into concentric rings around the eye socket, which is the center of the macula and the most sensitive area on the retina. Compared with the surrounding RPE, the RPE of the eye socket is a perfect hexagon, with more compact location and more adjacent cells.
Unexpectedly, they found that the peripheral retina contained a circle of RPE cells (P4), whose cell area was very similar to that in and around the macula.
"The presence of P4 subsets highlights the diversity around the retina, suggesting that there may be functional differences between RPES that we are not aware of at present," said Dr. Davide Ortolan, the lead author of the study and a researcher in the Department of eye and stem cell transformation of Nei. "Future research needs to help us understand the role of this subgroup."
Next, they analyzed RPE on cadavers with age-related macular degeneration (AMD). Due to the damage of the disease, the RPE of the orbital (P1) often does not exist, while the difference between the cells of the p2-p5 subgroup is not statistically significant. Overall, amd RPE subsets tend to elongate compared to RPE cells not affected by AMD.
To further test the hypothesis that different retinal degeneration affects specific RPE subsets, they analyzed ultra wide field fundus autofluorescence images of patients affected by choroidemia, l-ord or retinal degeneration with no identified molecular cause. Although these studies were conducted at one time point, they still show that different RPE subsets are vulnerable to different types of retinal degenerative diseases.
"Overall, these results suggest that AI can detect changes in RPE cell morphology before significant degradation and development," Ortolan said
Age related morphological changes may also occur in some RPE subsets and then be detected in other subsets. These findings will help provide information for future research using noninvasive imaging technologies, such as adaptive optics, which can solve the problem of retinal cells in unprecedented detail and may be used to predict RPE health changes in living patients.