Research shows that after the vaccination of Xinguan vaccine, with the extension of time, the immunity in the human body gradually weakens and the protection against virus infection decreases. After the vaccination of two doses of inactivated vaccine, the third dose of heterologous vaccine can induce a stronger immune response* Academician Chen Wei led the research and development of the world's first aerosol inhalation adenovirus vector recombinant coronavirus vaccine Ad5 ncov *, and conducted research to evaluate the safety and immunogenicity of the vaccine as the third dose of heterologous enhanced vaccine. The relevant results were recently published in the international authoritative academic journal Lancet respiratory medicine.

This is a randomized controlled, open label single center clinical trial. From September 14 to September 16, 2021, 420 adults who had been vaccinated with two doses of neocoronavirus inactivated vaccine were randomly assigned to the low-dose Ad5 ncov heterologous enhancement group (1:1) × 10 ^ 10 virus particles), high-dose Ad5 ncov heterologous enhancement group (2 × 10 ^ 10 virus particles), Kexing inactivated vaccine homologous enhancement group.
The main endpoints of safety and immunogenicity were the incidence of adverse reactions 14 days after vaccination and the geometric average titer of serum neutralizing antibodies.
The results showed that in terms of safety, after 14 days of inoculation, 26 patients (19%) in the low-dose group and 33 patients (24%) in the high-dose group had adverse reactions, which were significantly lower than 55 patients (39%) in the homologous enhancement group (P & lt; 0.0001) , but two subjects in the low-dose group and the high-dose group had grade 3 adverse reactions, including 2 cases of fever, 1 case of fatigue and 1 case of headache. There were no grade 3 adverse reactions in the homologous enhancement group.
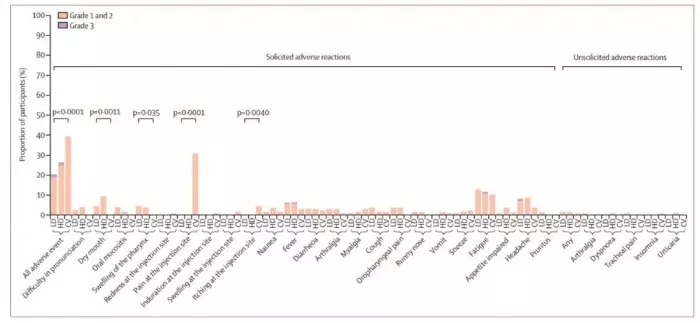
Adverse reactions of three groups of subjects: LD low-dose Ad5 ncov group, HD high-dose Ad5 ncov group and CV Kexing inactivated vaccine group
In terms of immunogenicity, after 14 days of inoculation, for the original sars-cov-2 strain, the average titer of serum neutralizing antibody in the low-dose group was 744.4 and 714.1 in the high-dose group, which were significantly higher than 78.5 in the homologous enhancement group (P & lt; 0.0001)**
The average titers of antibody against homologous strain 276.8 in the high dose group and 28.8 days in the high dose group were significantly higher than those against homologous strain 276.8 in the high dose group, respectively
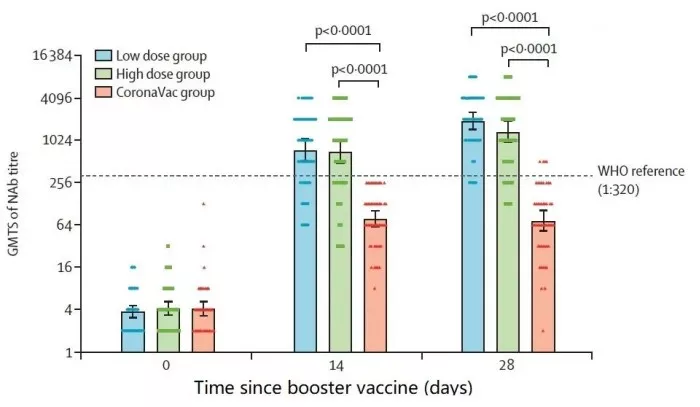
Geometric mean titers of neutralizing antibodies at different time points after vaccination in three groups of subjects
Compared with that before intensive vaccination, ELISPOT test showed that IFN- γ、 The expression level and increase multiple of IL-2 were significantly higher than those in the homologous enhancement group, suggesting that the specific T cell response to sars-cov-2 spike protein in the heterologous enhancement group was stronger than that in the homologous enhancement group.
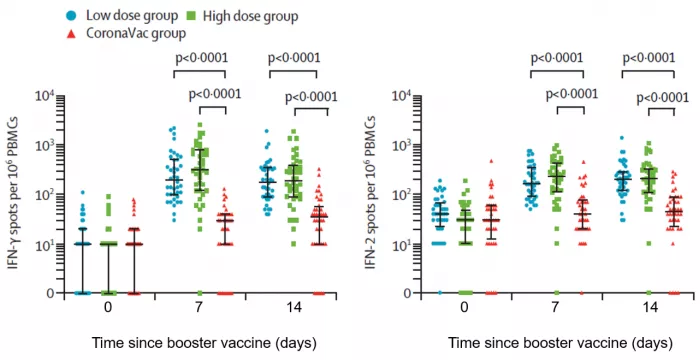
IFN of three groups of subjects at different time points after vaccination- γ And IL-2
The study showed that for adults inoculated with two doses of neocoronavirus inactivated vaccine, atomized inhalation of Ad5 ncov as the third dose of heterologous booster vaccine has good safety and high immunogenicity, and the effect is significantly better than that of homologous inactivated vaccine**