In the early 1980s, when AIDS came into people's sight as a terrible disease almost equal to the death penalty, perhaps few people could think that today, 40 years later, AIDS no longer means death, but has become a controllable chronic disease.
What does the emergence of the world's first female AIDS healer mean?
What changed all this was the emergence and development of antiretroviral therapy (Art). This kind of therapy can limit the HIV level in patients to a very low range and avoid symptoms. But these HIV carriers need lifelong treatment. Once they stop taking drugs, the HIV in their bodies will rebound rapidly. Moreover, long-term medication brings not only lifelong drug side effects, but also the risk of inducing drug resistance.
How to design long-term AIDS drugs so that patients can control the virus level within a safe range after drug withdrawal has become the next goal of scientists.
In the latest research in today's nature, the research team from the National Institute of allergy and infectious diseases has made a significant breakthrough. Through the combined use of two broad-spectrum neutralizing monoclonal antibodies, they confirmed the effect of long-term inhibition of HIV in a phase 1 clinical trial. This study is expected to provide an alternative to the current daily art treatment.

For a long time, scientists have been facing difficult obstacles when trying to clear the HIV reservoir in the body. Therefore, scientists have turned to another more feasible goal: with the help of long-term antiretroviral drugs or broad-spectrum neutralizing monoclonal antibodies (bnabs), to inhibit the viremia of HIV carriers, that is, to prevent the virus from spreading with the blood.
In recent years, the research on the treatment of AIDS with bnabs has made progress. This kind of antibody can target the envelope glycoprotein on the surface of HIV and prevent the virus from invading human cells. Bnabs drugs for the treatment of AIDS have also been available. However, people gradually realize that the effect of single use of bnabs is limited, because HIV in patients can evolve drug resistance. Therefore, to achieve this goal, at least two bnabs need to be used together.
This study used two bnabs that bind to different regions of the HIV envelope glycoprotein: 3bnc117 and 10-1074. Previous studies have verified the efficacy of these two antibodies in widely neutralizing HIV: 3bnc117 targets CD4, the main binding site of HIV invading the human body, and can play a role in more than 80% of HIV strains; While 10-1074 targets V3 glycan site, previous studies have also confirmed its high-efficiency broad-spectrum antiviral activity.
Using the combination of these two bnabs, the clinical trial designed by the research team is divided into two parts: the first is a randomized double-blind clinical trial. 14 people with acute or early HIV infection are divided into the experimental group and the control group. Among them, 7 subjects in the experimental group received 8 times of combined infusion of 3bnc117 and 10-1074 within 24 weeks. Soon after the first treatment, they stopped art treatment.
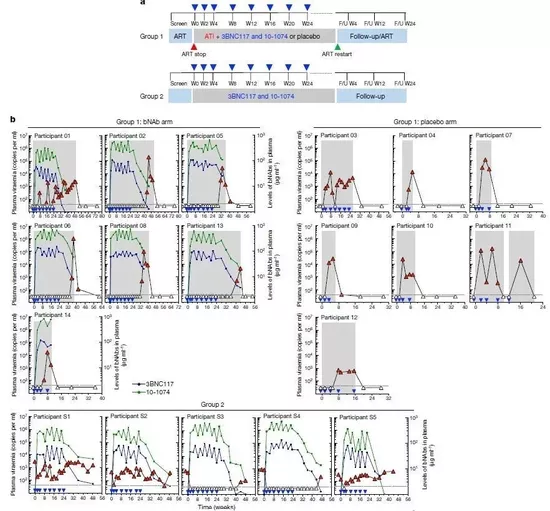
As a result, none of these subjects had serious adverse reactions. Compared with the control group, this combination therapy completely inhibited the viremia of HIV in 5 subjects in the experimental group, the longest inhibition effect reached 43 weeks, and no HIV resistant to antibodies was detected.
In addition, the second part of the study was a single arm, open label clinical trial. Five infected people who had not received art treatment but had a low level of HIV received the same combination therapy, and two of them achieved complete inhibition of viremia.
Combining these two trials, the research team confirmed that the combination therapy of broad-spectrum neutralizing monoclonal antibodies can inhibit the virus level for a long time without art treatment. This breakthrough also provides important guidance for the development of the next generation antibody with a long half-life. Next, larger and longer-term studies will further test the effectiveness of such combination therapies.
In addition, it should be pointed out that this therapy also has certain limitations: if the subjects have been resistant to one of the antibodies before the test, this bnabs combination cannot inhibit HIV. This also poses a major challenge for the development of future therapies.
"We have reason to believe that the long-term interval administration of such antibodies (e.g. twice a year), perhaps combined with long-term antiretroviral drugs, will help HIV infected people to suppress the virus in their bodies for a long time (for several years) without art treatment." The author said.