Recently, a piece of news continued to ferment. The new crown treatment drug recently discovered by Chinese scientists has been authorized by the national invention patent. According to the patent specification, 10um (micromol / L) stephanine inhibited coronavirus replication by 15393 times Professor tongyigang, the inventor of the patent and Dean of the school of life science and technology of Beijing University of chemical technology, said that a small amount of stephanine can prevent the expansion and spread of novel coronavirus. It can prevent the infection of the virus. Even after infection, it can reduce the virus to the level of 1 /10000 of normal infection

According to the current research data, the drug's ability to inhibit novel coronavirus ranks high among all novel coronavirus inhibitors found in humans.
Data show that stephanine is the active chemical component of Stephania, a traditional Chinese medicine. Chinese patent medicine has been listed in China and abroad for more than 40 years.
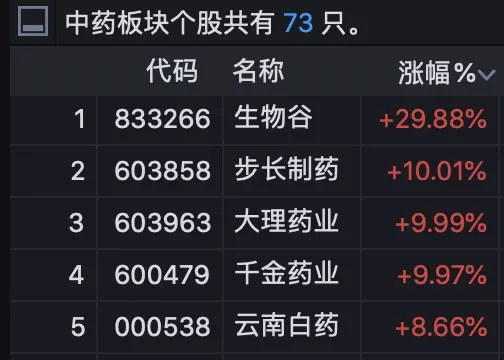
It is worth noting that on the day of news release, the A-share traditional Chinese medicine sector strengthened, and many stocks related to stephanine were active**
Biological Valley soared by 29.88%, up nearly 40% in the past five trading days. Step pharmaceutical, Huabei pharmaceutical, Dali pharmaceutical and Qianjin pharmaceutical rose the daily limit. Yunnan Baiyao and Aladdin once hit the daily limit, closing up 8.66% and 7.71% respectively.
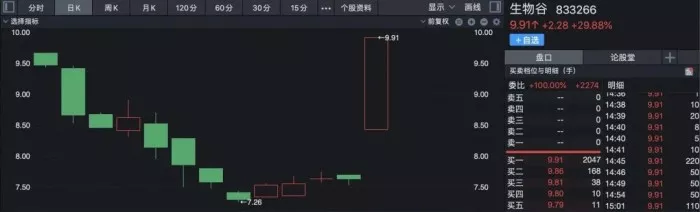
According to the public information, at present, the businesses of many companies such as step pharmaceutical, Yunnan Baiyao and North China Pharmaceutical have involved the field of stephanine**
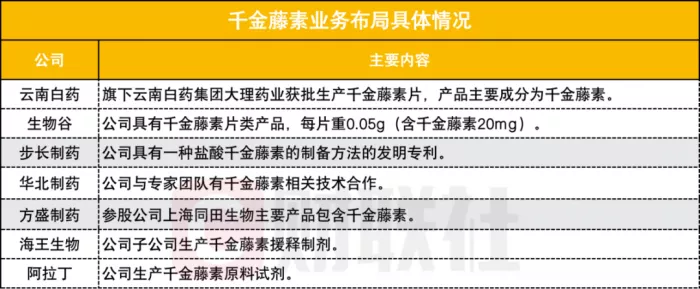
Qianjin pharmaceutical and Dali pharmaceutical seem to be "accidental" limit. Among them, Qianjin pharmaceutical was hyped by funds because its name contained "Qianjin". The company recently said on the interactive platform that there were no Qianjin related products. Dali pharmaceutical is due to the name collision with Dali Pharmaceutical Co., Ltd., a subsidiary of Yunnan Baiyao.
Soochow securities research report pointed out that under the control policy of dynamic clearing, Xinguan specific drugs are the key to long-term prevention and control in the future. At present, Pfizer paxlovid is the only qualified new crown specific drug approved for listing in China, and no domestic new crown specific drug has been approved for listing.
According to media reports, an American scholar previously published a paper in science confirming that the research data of stephanine is better than paxlovid, which has been approved for listing. At present, a Canadian pharmaceutical enterprise has contacted with the US FDA to carry out a clinical trial study on the treatment of COVID-19 with stephanine.
Moreover, some insiders estimate that the cost of stephanine is far lower than that of other drugs. At present, the market price of Pfizer paxlovid is 2300 yuan / box, and azdinf of real biology estimates 900 yuan / course of treatment, while referring to the price of old medicine of stephanine (old medicine and new use), it is about 100 yuan / box.
But at present, stephanine is still in the stage of drug discovery. Professor Tong Yigang also pointed out on the social platform that it will take some time for new drugs to reach the clinical and market. According to the data of new drug research and development in history, according to the analysis of market participants, the probability of success of stephanine is about 3.1%
It is worth noting that the patent was applied in August 2021 and has not entered animal experiments in the past year.
A medical professor who is also engaged in the research and development of new crown drugs said that the mechanism of action of stephanine against novel coronavirus is not clear. It depends on the antiviral activity of the drug in vitro, and it needs to be compared with the drugs already on the market or under clinical development. It needs to use the same test method for comparison.
Xinguan oral drug clinical trial approved! The application for Chinese medicine to double bull stocks was accepted after four days, and then the profit was good, and the institution fled at a high level
Zhongsheng pharmaceutical, a traditional Chinese medicine stock with a total market value of over 14billion yuan, announced in the afternoon that the clinical trial of Zhongsheng RuiChuang, a holding subsidiary, oral anti novel coronavirus 3CL protease inhibitor ray1216 tablets was approved by the State Drug Administration.

As soon as the announcement was made, investors debated on the app of Cailian one after another. Some investors said, "cow, it's a word board". Some investors said, "cut it, come here.".
Four days ago, Zhongsheng pharmaceutical announced after hours on May 11 that the application for clinical trial registration of the holding subsidiary Zhongsheng RuiChuang oral anti novel coronavirus 3CL protease inhibitor class innovative drug ray1216 tablets was accepted by the State Drug Administration.

In terms of the secondary market, after Zhongsheng pharmaceutical issued a favorable announcement after three hours last week, the share price opened high on Thursday, and then closed the daily limit; However, it fell sharply after rising on Friday, closing down nearly 6%, with a cumulative increase of 3.85% in two trading days; For a long time, the stock price of Zhongsheng pharmaceutical has increased by 157% since its low point in February last year.
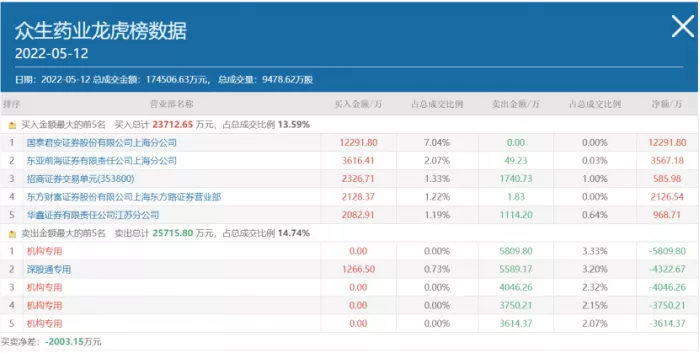
It is worth noting that the one-day list data released by Zhongsheng pharmaceutical on Thursday showed that the funds of the dragon and tiger list were sold for a net of 2003 million yuan. Among them, institutional funds were sold on a large scale, ranking first, third to fifth, with 58.09 million yuan, 40.46 million yuan, 37.5 million yuan and 36.14 million yuan sold respectively; Shenzhen Stock connect ranks as the second selling seat.
Public information shows that the traditional business of Zhongsheng pharmaceutical is the production and sales of Chinese patent medicine, and the field of chemical medicine will be gradually arranged after listing. After the outbreak of COVID-19 in early 2020, the company started the research and development of anti novel coronavirus drugs, and independently developed a broad-spectrum anti Xinguan oral drug with global independent intellectual property rights, namely ray1216.
Zhongsheng pharmaceutical ray1216 blocks virus replication through anti novel coronavirus 3CL protease inhibitor, so as to achieve the effect of anti novel coronavirus. It is worth noting that Pfizer paxlovid and yanyeyi Xinguan drug s-217622 are Xinguan oral drugs targeting 3CL targets.
Zhu Guoguang, an analyst at Soochow securities, said in the Research Report on May 15 that the rapid progress of domestic Xinguan small molecule oral drugs is the key to epidemic prevention and control. Xinguan small molecule oral drug is less affected by the variation of the virus strain, and has the advantages of low production cost and convenient administration. It has great potential in the prevention and control of COVID-19.
From the current domestic xinguankou drug market, according to the Research Report of BOC securities, azvudine of real biology, vv116 of Junshi biology and pukelu, which develops the pharmaceutical industry, belong to the first echelon of the industry and are in clinical phase III. Xiansheng pharmaceutical and cutting-edge biology are also in clinical phase I.
In addition, the company also issued another announcement in the afternoon that the first subject of the phase III clinical trial of the subsidiary's class I innovative drug zsp1273 tablet has been included in the group. Zsp1273 tablet is intended to be used for the prevention and treatment of influenza A and human avian influenza. It is the first small molecule RNA polymerase inhibitor for the treatment of influenza A approved and completed phase I and II clinical trials in China.