Many studies on Alzheimer's disease have cited the accumulation of cerebral plaques as the main cause, but this case is far from over, especially in the view of a research team at New York University. In the newly published study, scientists have detailed how the decline in acidity of cell cleaning organelles called lysosomes can serve as early evidence of the onset of the disease , and they have shown that restoring appropriate acidity levels can save neurons from irreversible damage.
β- The accumulation of amyloid in the brain leads to the neurodegeneration of Alzheimer's disease. This concept has existed for decades and has guided most of the research on potential treatment. However, in recent years, some promising drugs developed for these plaques have failed. Although the amyloid hypothesis is still a very active branch of Alzheimer's disease research, some people are looking for answers elsewhere, and those who study lysosomal function are finding some very valuable insights.
Lysosomes are organelles found in the cells of many animals, which contain acid enzymes for decomposition, removal and recycling of waste. For this reason, they are called cellular garbage disposal systems, and recent studies have begun to show how the dysfunction of this system plays a role in the neuronal damage seen in Alzheimer's disease.
An interesting study in 2019 showed that amyloid proteins can reverse their molecular structure, making lysosomes unable to recognize them and clear them. The resulting accumulation of failed lysosomes is believed to contribute to neuronal damage. This study supports a 2015 Yale University study that showed that this can enhance the accumulation of toxic proteins at the same time.

This new study adds further weight to this idea by demonstrating how lysosomal dysfunction can drive neuronal damage before amyloid plaques are fully formed. The authors used mice bred to develop Alzheimer's disease and tracked the acid content in lysosomes because cells were injured by the disease.
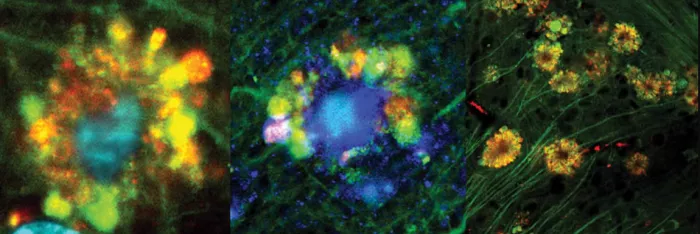
Lysosomal imaging showed that the acidity level decreased with the injury of neurons. Some of these lysosomes became larger because they interacted with vesicles filled with waste that could not be decomposed, causing vesicles to gather into flower shaped ridges around the cell membrane. Some of these vesicles were found to contain early forms of β- Amyloid proteins, which continue to form filaments in cells and develop into fully formed plaques in some damaged neurons.
Dr. Ju Hyun Lee, lead researcher of the study, said: "our results are the first to attribute the neuronal damage observed in Alzheimer's disease to problems within the lysosome of brain cells, where β- Amyloid protein appeared first. Previously, the work hypothesized that most of the damage observed in Alzheimer's disease was attributed to the accumulation of amyloid protein outside the brain cells, rather than before and from within the neurons. "

Scientists are now working to develop treatments for this form of lysosomal dysfunction. In their previous work, they linked lysosomal disorders to PSEN1, a gene long associated with Alzheimer's disease. Now they have been able to demonstrate how this form of neuronal damage is reversed by restoring lysosomal acidity to normal levels.
"This new evidence has changed our basic understanding of how Alzheimer's disease progresses; it also explains why many experimental therapies aimed at clearing amyloid plaques have failed to prevent the progress of the disease, because brain cells are incomplete before the plaques are completely formed outside the cells," said Ralph A. Nixon, senior investigator of the study. "Our research suggests that future therapies should focus on reversing lysosomal dysfunction and rebalancing acid levels in brain neurons."
The study was published in Nature Neuroscience 》In magazines.