The National Institute of allergy and infectious diseases (NIAID) under the National Institutes of Health (NIH) has launched an early clinical trial to evaluate an investigational preventive vaccine against Epstein Barr virus (EBV). EB virus is the main cause of infectious mononucleosis ("mono") and is associated with some cancers and autoimmune diseases. This phase 1 study is one of the only two studies to test investigational EB virus vaccine in more than a decade. It will be conducted at the NIH Clinical Center in Bethesda, Maryland.
EBV is a member of herpesvirus family and one of the most common human viruses. It is transmitted through body fluids, most notably saliva. In the United States, there are an estimated 125000 cases of infectious mononucleosis each year; About 10% of them experienced fatigue lasting 6 months or more. About 1% of EBV infected people have serious complications, including hepatitis, neurological problems or serious blood abnormalities. EBV is also associated with several malignancies, including gastric and nasopharyngeal carcinoma, Hodgkin's and Burkitt's lymphoma, as well as autoimmune diseases such as systemic lupus erythematosus and multiple sclerosis.
Anthony S. Fauci, M.D., director of NIAID, said: "a vaccine that can prevent or reduce the severity of Epstein Barr virus infection can reduce the incidence rate of infectious mononucleosis, and may also reduce the incidence rate of EB virus related malignant tumors and autoimmune diseases."
Under the leadership of Jessica Durkee shock, M.D., the principal investigator of NIAID infectious diseases laboratory, the study will evaluate the safety and immune response of an EBV gp350 ferritin nanoparticle vaccine under study and use a saponin based matrix-m adjuvant. The experimental vaccine was developed by the infectious diseases laboratory in cooperation with the vaccine research center of NIAID. Matrix-m adjuvant was developed by Novavax, a biotechnology company based in Gaithersburg, Maryland.
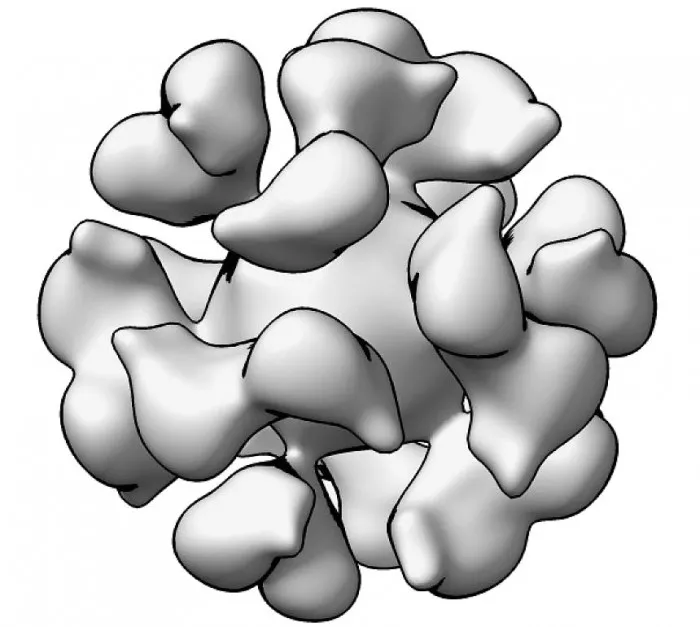
The vaccine works by targeting EBV glycoprotein gp350, which exists on the surface of viruses and virus infected cells. EBV gp350 is also the main target of neutralizing antibodies found in the blood of people naturally infected with EBV. Ferritin is a natural ferritin found in cells of all biological species. It is considered to be a promising vaccine platform because it can display proteins from target viruses densely on its surface. Adjuvants are designed to enhance the immune response induced by research vaccines.
The study will recruit 40 healthy adult volunteers aged 18 to 29, half of whom have evidence of previous EBV infection and half have no evidence of previous EBV infection. Participants will be injected with 50 micrograms of experimental vaccine in the upper arm muscle three times and observed for 30 to 60 minutes after each injection. The second and third injections will be performed 30 and 180 days after the initial dose, followed up between each vaccination and telephone contact between the two follow-up. It is expected to take 18 to 30 months, and the test is expected to last for four years.
More information about this study can be found at ClinicalTrials The identifier nct04645147 on gov is available.