The researchers found that a molecular gel point and a timely turning point helped a bacterial enzyme quickly convert carbon dioxide into carbon compounds, 20 times faster than plant enzymes in photosynthesis This result will accelerate the progress of converting carbon dioxide into various products.
Carbon fixation, or converting carbon dioxide in the air into biological macromolecules rich in carbon, is very important for the survival of plants. This is the full meaning of photosynthesis and the cornerstone of the huge chain system of carbon cycling through plants, animals, microorganisms and the atmosphere to maintain life on earth.
However, the "champion" of carbon sequestration is soil bacteria, not plants. If scientists can figure out how some bacterial enzymes are an important step in carbon fixation 20 times faster than plant enzymes, they may be able to develop artificial photosynthesis to convert greenhouse gases into fuels, fertilizers, antibiotics and other products.
Now, a research team from the SLAC National Accelerator Laboratory of the U.S. Department of energy, Stanford University, the Max Planck Institute of terrestrial microbiology in Germany, the joint Genomics Institute (JGI) of the U.S. Department of energy and the University of Concepcion in Chile has found how a bacterioenzyme -- a molecular machine that promotes chemical reactions -- is activated to accomplish this feat.
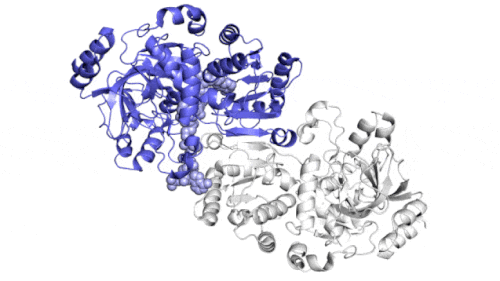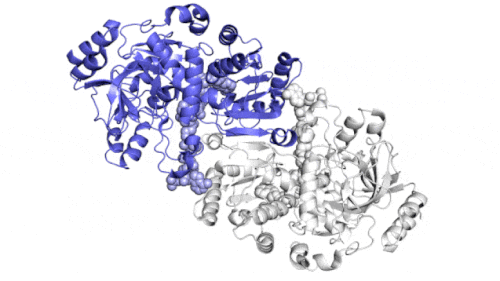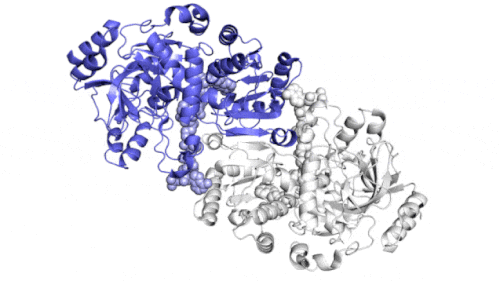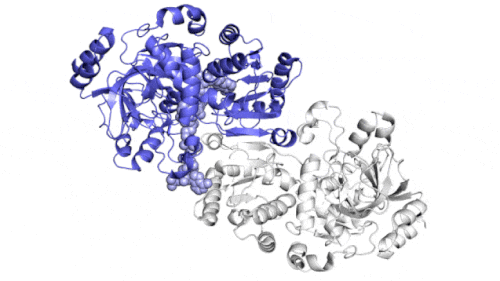
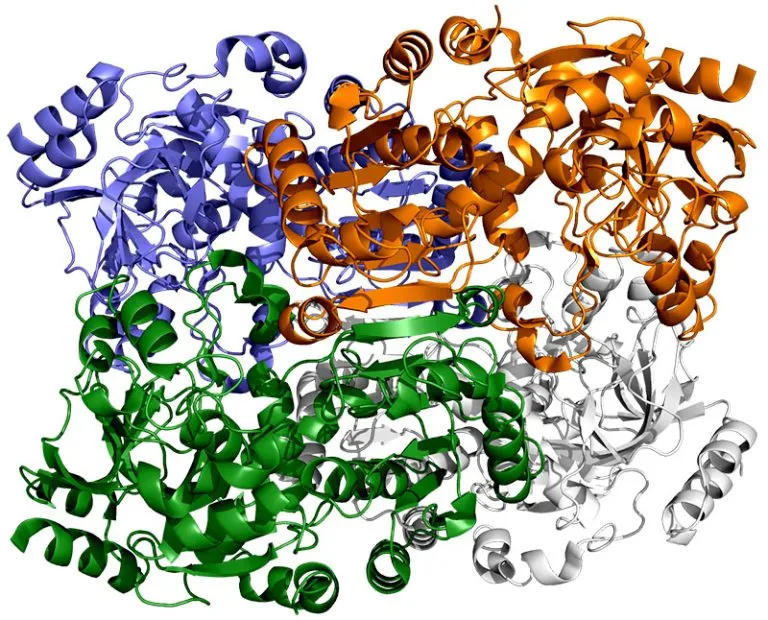
They found that instead of grabbing carbon dioxide molecules and attaching them to biological macromolecules one by one, the enzyme consists of a pair of molecules that work synchronously, just like the hands of a juggler who throws and catches a ball at the same time to complete the work faster. One "member" in each pair of enzymes opens to capture a group of reaction components, while the other "member" closes its captured components and carries out carbon fixation reaction; They then switch roles in a continuous cycle.
The team found that a molecular "glue" holds the hands of each pair of enzymes together so that they can open and close alternately in a coordinated manner, while the twisting movement helps to "drive" the ingredients and finished products out of the reacting pockets. When both glue and twist are present, the carbon fixation reaction is 100 times faster than without them.
"This bacterial enzyme is the most effective carbon fixator we know, and we have come up with a complete explanation of what it can do," said Soichi wakatsuki, a professor at SLAC and Stanford University and one of the senior leaders of the study, which was published this week in ACS Central Science 》Come on.
"Some enzymes in this family act slowly, but in a very specific way, they produce only one product. Others are much faster and can make chemical components for various products. Now that we know the mechanism, we can design enzymes that combine the best characteristics of these two methods and process various starting materials very quickly," he said
Improve on the basis of nature
The enzymes studied by the team are part of a family called enoyl CoA carboxylase / reductase, or ECRs. It comes from soil bacteria called kitasatospora setae, which can produce antibiotics in addition to their carbon sequestration skills.
Six months ago, wakatsuki heard about this enzyme family from Tobias Erb of Max Planck Institute for terrestrial microbiology in Germany and Yasuo Yoshikuni of JGI. Erb's research team has been committed to developing bioreactors for artificial photosynthesis to convert carbon dioxide in the atmosphere into various products.
Erb said that although photosynthesis is important for life on earth, it is not efficient. Like all things formed in the long process of evolution, it can only do its best. This is the result of slowly building on previous development, but nothing new has ever been revealed from the hair.
More importantly, he said, the step of fixing carbon dioxide from the air in natural photosynthesis, relying on an enzyme called Rubisco, is a bottleneck, putting the whole photosynthetic reaction chain into trouble. Therefore, using a fast ECR enzyme to perform this step and making it faster through engineering design can bring a significant improvement in efficiency.
"We're not trying to make a carbon copy of photosynthesis," Erb explained. "We want to use our understanding of engineering to reconstruct the concept of nature and design a more effective process. This' photosynthesis 2.0 'can be carried out in living or synthetic systems, such as artificial chloroplasts - water droplets suspended in oil."
A portrait of an enzyme
Wakatsuki and his team have been working on a related system, nitrogen fixation, which converts nitrogen in the atmosphere into compounds needed by organisms. He was curious about why ECR enzyme was so fast and began to work with Erb's team to find the answer.
Hasan demirci, a research assistant in wakatsuki's team, is now an assistant professor at Koc University and an investigator at Stanford pulse Institute. With the help of half a dozen SLAC summer interns he supervised, he led the work of SLAC. "We train six or seven of them every year. They are fearless. They come with an open mind and are ready to learn. They have done amazing things," he said
The SLAC team made samples of ECR enzymes and crystallized them with X-rays at the advanced photon source at the Argonne National Laboratory of the U.S. Department of energy. X-rays revealed the molecular structure of the enzyme - the arrangement of its atomic scaffolds - both in itself and when connected to a small helper molecule that promotes its work.
Further X-ray studies at SLAC's Stanford synchrotron radiation light source (SSRL) show how the structure of the enzyme changes when it is connected to the substrate, which is a molecular workbench used to assemble the components of the carbon fixation reaction and promote the reaction.
Finally, a research team from Lincoln coherent light source (LCLs) of SLAC studied the enzyme and its substrate in more detail on sacla X-ray free electron laser in Japan. Choosing an X-ray laser is important because it allows them to study the behavior of the enzyme at room temperature - closer to its natural environment - with little radiation damage.
At the same time, the ERB group in Germany and the Esteban VO ¨ hringer Martinez associate professor group at the University of Concepcion in Chile conducted detailed biochemical studies and extensive dynamic simulations to understand the structural data collected by wakatsuki and his team.
Wakatsuki said that the simulation results showed that the opening and closing of the two parts of the enzyme involved not only molecular gluing, but also twisting movement around the central axis of each enzyme pair.
"This twisting is almost like a ratchet that can push a finished product out or pull a new set of ingredients into the reacting pocket. The twisting and synchronization of enzyme pairs enable them to fix carbon 100 times in a second," he said
The ECR enzyme family also includes a more general branch, which can interact with many different kinds of biological macromolecules to produce various products. But because they are not held together by molecular "glue", they cannot coordinate their movement, so they operate much slower.
"If we can speed up these complex reactions to create new biological macromolecules, it will be a major leap in this field," wakatsuki said
So far, these experiments have produced static snapshots of various configurations of enzymes, reaction components and final products.
"Our dream experiment is to combine all the components as they flow into the path of the X-ray laser beam so that we can observe the reaction in real time," wakatsuki said
He said the team actually tried this at sacla, but failed. "Carbon dioxide molecules are really small, and they move so fast that it's hard to capture the moment they attach to the substrate. Plus, the X-ray laser beam is so powerful that we can't keep the components in it long enough to react. When we press hard, we try to break the crystal," he said
He added that the upcoming high-energy upgrade of LCLs may solve this problem. The frequency of pulse arrival is much higher - one million times per second - and can be adjusted separately to the ideal intensity of each sample.
Wakatsuki said his team will continue to work with Erb's team and with researchers from the LCLs sample delivery team and SLAC Stanford cryo electron microscope (cryo EM) facility to find a way to make this method work.
Researchers from the SPring-8 center of the Japan Institute of physics and chemistry and the Japan Institute of synchrotron radiation also contributed to this work, which was mainly funded by the science office of the U.S. Department of energy. Most of the preliminary work of this study was completed by Yash Rao, SLAC summer intern; Interns Brandon Hayes, e. Han Dao and manat Kaur also made important contributions. DOE's joint Genomics Institute provided DNA for the production of ECR samples. SSRL, LCLs, advanced photon source and joint Genomics Institute are user facilities of DOE science office.
