Since the outbreak of the new coronavirus outbreak in 2020, 'nucleic acid testing' has begun to recur as an important indicator to detect the presence of infection. 2023 The decision was made to promote the "antigen screening, nucleic acid diagnosis" testing model, adding antigen testing as a supplement to nucleic acid testing.
What is an antigen test? How is it different from other tests? In this article, we talk about common rapid screening tools, the principles involved and the scope of application, using the novel coronavirus as an example.
When we do screenings, what exactly can we look for
To screen for a disease or a substance, the first thing we need to figure out is the question of 'where to start', followed by 'how to detect', so that the changes in the microscopic world are reflected in front of our eyes and help us make a judgment.
Where to start
We are dealing with viruses. According to familiar knowledge from secondary school biology classes, viruses are a class of life-like organisms consisting of genetic material and a protein shell. If you want to detect the infection of a virus, you need to start with its components. For what follows, I hope you will bring your secondary school biology knowledge to read.
Take the example of SARS-CoV-2, which is currently plaguing us. It belongs to the genus B coronavirus of the subfamily Coronaviridae, under the family Coronaviridae, and is the seventh known coronavirus capable of infecting humans. All coronaviruses are just single-stranded RNA viruses with envelope, that is, their genetic material is a single RNA strand, and this RNA strand can directly participate in translation as mRNA (messenger RNA) to direct protein synthesis.
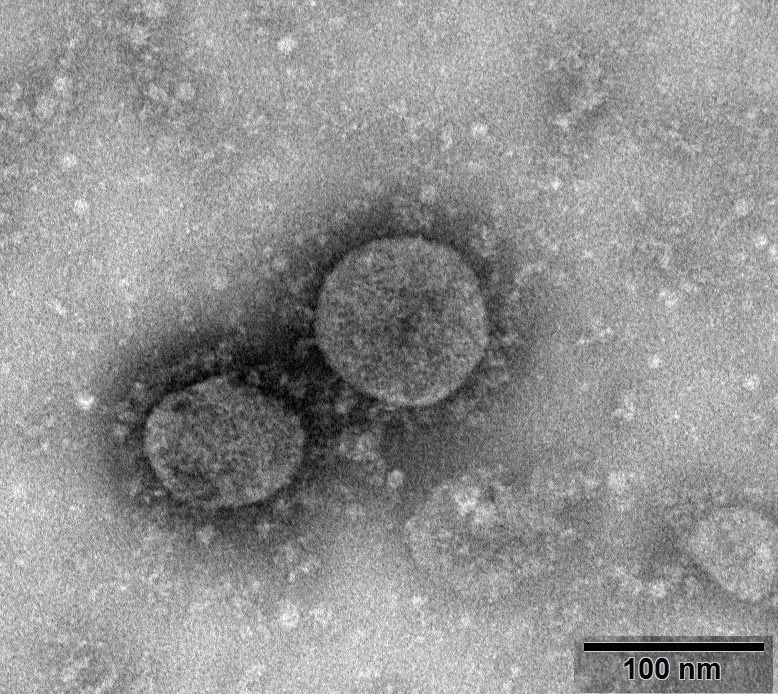 Virulent species with the number NPRC 2020.00002, image courtesy of the National Pathogenic Microorganism Repository (Chinese Center for Disease Control and Prevention, Viral Disease Prevention and Control Institute). Source: https://nmdc.cn/nCoV Our aim now is to detect the presence of this virus in the specimen, either by detecting it itself or by detecting the products brought by the virus, and there are two directions in which we can start: the protein shell (envelope), and the genetic material.
Virulent species with the number NPRC 2020.00002, image courtesy of the National Pathogenic Microorganism Repository (Chinese Center for Disease Control and Prevention, Viral Disease Prevention and Control Institute). Source: https://nmdc.cn/nCoV Our aim now is to detect the presence of this virus in the specimen, either by detecting it itself or by detecting the products brought by the virus, and there are two directions in which we can start: the protein shell (envelope), and the genetic material.
How to detect
Following this logic, then the most obvious way is to check whether it "can be seen", but the virus itself is so small, SARS-CoV-2 is 80-120 nm in diameter, that it is impractical to take every specimen through the electron microscope, and the human and financial resources cannot sustain it. A more cost-effective method, then, is to allow component of the virus, or certain special substances that appear because of the virus, to accumulate to a certain order of magnitude and then glow, change color, and appear macroscopic manifestations.
Our problem then translates into choosing a way to observe macro-scale changes, to associate with a component of the virus, a substance that the virus triggers. The substances we can choose are also on the table: the genetic material of the virus, in this case its RNA; the envelope of the virus, the protein shell; and, if you remember some basic biology, the body's immune system produces antibodies to fight off invasion after infection with a virus, and it's a good choice.
Several of the tests we currently use are derived from these substances (and their related substances), namely nucleic acid tests for genetic material, antigen tests for envelopes, and serum antibody tests for antibodies.
Nucleic acid testing
As the genetic material of a virus, the nucleic acid sequence contains the genetic features that can identify the virus as a particular species, so a positive nucleic acid also means that the virus was present in the body.
The "nucleic acid test" we are currently performing is actually divided into two parts. The usual "nose poke" and "throat poke" sampling and subsequent characterization we do is the first part. After obtaining the specimen, because the amount of virus is so small, the sample is amplified a certain number of times in the lab and a positive negative is determined based on the fluorescence reaction results.
In the second part, samples identified as positive also need to be genetically sequenced to determine the typing of the sample virus for traceability. This step is no longer part of routine screening, but is of great importance in epidemiological investigations and can be briefly described in Wikipedia if you are interested.
The nucleic acid test that we normally participate in as a screening tool refers to the first part of the collection to characterization.
Following infection with SARS-CoV-2, viral nucleic acids can be found in specimens such as throat swabs, sputum, lower respiratory secretions, and blood. The positive rate of nucleic acid detection in different parts of the specimen varied somewhat, and the rate of detection in each site changed as the disease progressed.
Both "nasal swabs" and "pharyngeal swabs", as we are used to calling them, are in fact the collection of secretions and tissues from the posterior wall of the pharynx, the former from the nasopharynx and the latter from the oropharynx. There are also other standards, such as saliva, which can also be used as specimens for testing, but essentially there are also differences in regulations in different regions
Nasal (pharyngeal) swabs and (oropharyngeal) swabs are already a combination of positive rates and convenience considerations. Stool and urine, for example, can actually be used for specimen collection. Furthermore, according to a study of 31 patients, anal swabs were more accurate than nasopharyngeal and oropharyngeal sampling, with less than 30% of nasal swabs being positive in cases confirmed by anal swabs, especially late in the course of the disease. However, it is clear that it cannot be used as a preferred means of early screening because of operational limitations.

 The next task was to extract the nucleic acids from the little bit of specimen that was obtained. Since the order of magnitude of virus in the sample is too small to bring to analysis, it also needs to be amplified and labeled. This also requires the same knowledge you learned in high school: polymerase chain reaction (PCR) - this step may seem like a hassle, but since its principles and procedures have been well studied, in practice all you need to do is add the reagents and send them to the machine, and the most troublesome part of the whole nucleic acid testing process is getting the specimen to the test taker (laughs).
The next task was to extract the nucleic acids from the little bit of specimen that was obtained. Since the order of magnitude of virus in the sample is too small to bring to analysis, it also needs to be amplified and labeled. This also requires the same knowledge you learned in high school: polymerase chain reaction (PCR) - this step may seem like a hassle, but since its principles and procedures have been well studied, in practice all you need to do is add the reagents and send them to the machine, and the most troublesome part of the whole nucleic acid testing process is getting the specimen to the test taker (laughs).
Each local CDC or testing center will purchase the appropriate nucleic acid extraction kit with the nucleic acid detection kit. The extraction kit is responsible for extracting the RNA from the mixed sample (cell debris, secretions, dust, and other impurities), commonly by magnetic beads, centrifugal columns, and releasers. The purified RNA is then handed over to the assay kit (or some kits combine the two) for the subsequent process.
The process in which the test kit carries the sample through the machine is the primary reaction in this test: RT-qPCR (real-time quantitative reverse transcription polymerase chain reaction).
The next step requires you to pick up your knowledge of high school biology. A typical PCR reaction has the following steps.
- (a) Heat: to deconvolute double-stranded DNA into two single-stranded strands.
- Annealing: allowing the mixed single-stranded DNA to bind to primers designed according to the fragment to be replicated.
- Extension: adjust the temperature to allow the DNA polymerase to start working down the primer, copying the new strand and forming a new double strand.
In the detection of viruses, we have to do no more than the above steps, except that two additional things are needed.
- Synthesis of complementary strands of viral single-stranded RNA, assembled into cDNA, using reverse transcriptase (RNA-dependent DNA polymerase) prior to the first reaction.
- During the annealing and extension phase, in addition to the primers and the required enzymes, TaqMan probes are required.
You can think of the TaqMan probe like this: its main part is a piece of oligonucleotide strand designed to pair up with a small portion of the gene fragment to be replicated as a double strand; it has a fluorescent molecule attached to one end and a switch (quenching group) attached to the other end, and when both are attached to the probe, the fluorescence is suppressed by the quenching group and not detected. When annealed, this probe binds to the single-stranded fragment to be replicated along with the primer. During extension, the DNA polymerase shreds the obstacles in its way, which include this segment of the probe, and the quenched group and the fluorescent molecule are thus separated and the fluorescence is expressed.
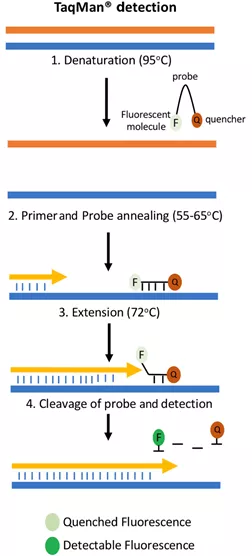 Figure credit: Grace Adams; A beginner's guide to RT- PCR, qPCR and RT-qPCR. Biochem (Lond) 22 June 2020; 42 (3): 48-53. doi: https://doi.org/10.1042/BIO20200034 As the number of cycles increases, the number of amplified DNA fragments and fluorescence also increase. Comparing the fluorescence brightness of each cycle with the baseline brightness of the previous cycles gives us the current amount of DNA fragments, or we can use the cycle number and fluorescence brightness directly to make a qualitative determination.
Figure credit: Grace Adams; A beginner's guide to RT- PCR, qPCR and RT-qPCR. Biochem (Lond) 22 June 2020; 42 (3): 48-53. doi: https://doi.org/10.1042/BIO20200034 As the number of cycles increases, the number of amplified DNA fragments and fluorescence also increase. Comparing the fluorescence brightness of each cycle with the baseline brightness of the previous cycles gives us the current amount of DNA fragments, or we can use the cycle number and fluorescence brightness directly to make a qualitative determination.
So which part exactly is replicated? Since we are detecting the virus, we will pick the most representative nucleic acid fragment. The ORF gene and the N gene are the commonly used detection sites in the current standard.
The test kit is responsible for inputting the extracted RNA (sample), setting the corresponding PCR temperature and duration according to the program instructions on the kit, and having the machine control complete the amplification process, collecting the fluorescence signal at a fixed session and recording the corresponding cycle count (Ct value). The criterion for determining if a negative positive / is still infectious is to see what the current cycle count is when the fluorescence signal reaches the threshold value. According to the current "Novel Coronavirus Pneumonia Treatment Protocol (Trial Version 9)", the criteria for release from isolation management is Ct value ≥ 35. Compared to the previously prevailing standard of ≥40, the time between discharge and discharge from isolation will be significantly reduced.
immunoassay
Nucleic acid testing by RT-PCR has been the gold standard for confirming diagnosis until now, as it is (theoretically) 100% accurate from a methodological point of view. However, nucleic acid testing is time-consuming and demanding on the environment and operators, and large volumes of nucleic acid testing can be a huge drain on human and financial resources when environmental conditions are not up to standard and supplies and instruments are not available.
The immunoassay we used in this outbreak included a rapid antigen assay and an antibody assay, which is based on the basic principle of antigen-antibody reaction and is designed to detect the presence of the substance to be tested in a sample at less time cost through the rapid neutralizing effect of antigen and antibody. Both fall under the category of immunochromatographic assays.
Antigen tests that come in cartridges and can be performed on their own are well suited as a supplement in cases of low supplies, self-assays, etc.
Antigen detection
The nucleic acid test examines the (signature) genetic material of the virus, the 'insides' of the virus. The (rapid) antigen test then examines the 'outside' of the virus, directly examining the intact virus particles. Currently there are three types of approved antigen testing kits: colloidal gold, latex, and fluorescence immunochromatography. All three have the same internal principle. However, fluorescence immunochromatography kits still require a special detector or UV flashlight and are not suitable for home self-testing; colloidal gold and latex methods both translate the test results into visible bands for the naked eye, but the difference lies in the substance used to mark the coloring.
Of course, antigen testing naturally has its disadvantages in that it has a higher rate of false negatives (being positive but showing negative), which can lead to missed tests and wrong tests. But put in front of the time advantage of having results in a cup of tea, the accuracy gap can temporarily give way in some specific cases.
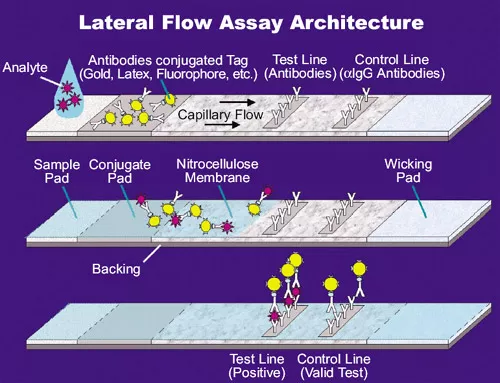 Figure source: How the SARS-CoV-2 EUA Antigen Tests Work | ASM.orgCompared to nucleic acid testing, antigen testing adds a 'nasal swab' as a sampling route, making it less difficult for individuals to self-test. The sample on the swab is eluted in buffer, and after a drop of liquid is taken and added to the spiked well, the liquid carries the potential antigen, due to capillary action, through an area preloaded with antibodies (the conjugate pad).
Figure source: How the SARS-CoV-2 EUA Antigen Tests Work | ASM.orgCompared to nucleic acid testing, antigen testing adds a 'nasal swab' as a sampling route, making it less difficult for individuals to self-test. The sample on the swab is eluted in buffer, and after a drop of liquid is taken and added to the spiked well, the liquid carries the potential antigen, due to capillary action, through an area preloaded with antibodies (the conjugate pad).
The antibodies on this area, which are monoclonal antibodies against the target antigen (SARS-CoV-2), each antibody molecule is bound to a special marker, and they react with the antigen in the sample to form an antigen-antibody complex that flows with capillary action to the next band.
Immediately afterwards, you pass the test line, where the same monoclonal antibody against the target antigen is attached, which you can interpret as the same thing as on the binding pad, only without the label. At this point, if the test subject has been infected with SARS-CoV-2, the antigen-antibody complexes formed by the antigen he left in the sample will bind again here to the antibodies fixed on the line. Here, these labelled complexes are continuously deposited and will eventually show a dark or light band. The source of the color of the strip is the marker attached to the antibody molecules on the previously bound pad, in the colloidal state of gold particles in the colloidal gold method, colored latex droplets in the latex method, and fluorescent molecules in the fluorometric method. So you'll notice when you're using these types of test strips that just after you've finished spiking the sample and the liquid is just starting to diffuse, there's a little bit of very faint color at the very front of the diffusion that keeps pushing through, and that's the color of the marker that hasn't been fixed and deposited yet.
Next, the liquid continues to diffuse, passing through the quality control line (C line, control line), where another antibody is attached - 'a monoclonal antibody against "a monoclonal antibody against the target antigen", or "diatomic antibody" for short. This new antibody is obtained by having the previous antibody react in the immune system of another animal; for example, if the antibody to the binding pad is from a rabbit, then the antibody here is from a sheep, a monoclonal antibody to a sheep anti-rabbit. That is, the antigen of the secondary antibody is the antibody previously on the binding pad. This line is there to detect if the fluid has spread properly, if the antibodies on the binding pad have failed, etc. At this point, the large amount of antibody from the binding pad remaining in the liquid acts as an antigen and reacts with the secondary antibody on the QC line to form a complex, showing a distinct band.
Because the binding pad is so full of antibodies, this QC line band will appear very quickly and colorfully, while the detection line will vary in the speed of color development due to the number of antigens (viruses), but it is usually sufficient to determine the result within 15 minutes. So don't look at the C line as obvious and the T line as faint and think "it's okay", The T line is positive regardless of the shade.
For details on the operation, you can refer to the instructional video published by the Medical and Health Administration. The state is also gradually promoting antigen self-testing kits, which will, to some extent, reduce future medical and street pressure.
Serum antibody test
In addition to the first two tests, another less used but equally important test is the "serum antibody test", which is also an immunoassay.
The kit used for the antibody test is very close to the antigen test, but the specimen is much more limited - since the test becomes for antibodies, the specimen must then be blood (or plasma or serum) that clearly has antibodies present. And the body does not produce antibodies within the first hour after initial infection with the virus. The antibodies can clearly reach the order of magnitude being tested, usually one to two weeks after the initial infection (or vaccination). These conditions limit the use of antibody testing as a test of a confirmatory nature. Currently, serum antibody testing is only used as a method to check for vaccine efficacy under certain circumstances or to check whether the subject has recently been infected with a new coronavirus.
There are five types of antibodies in the body, IgA, IgD, IgE, IgG and IgM. IgA is mainly responsible for mucosal immunity, IgD is associated with the activation of the immune response, IgE fights parasites and is also involved in allergic reactions. The remaining IgG and IgM are the mainstays of the immune system in the fight against pathogens.
As a pathogen, SARS-CoV-2 is mainly secreted by the body upon stimulation with IgG and IgM. The existing antibody test kits mainly target the IgG and IgM produced by the body in response to the N protein (nucleocapsid protein) or S protein (stinging protein) of SARS-CoV-2.
The appearance of the detection device of the antibody kit is not different from that of the antigen test. The difference between the two is the substance attached to the binding pad, detection line, and quality control line as mentioned above.
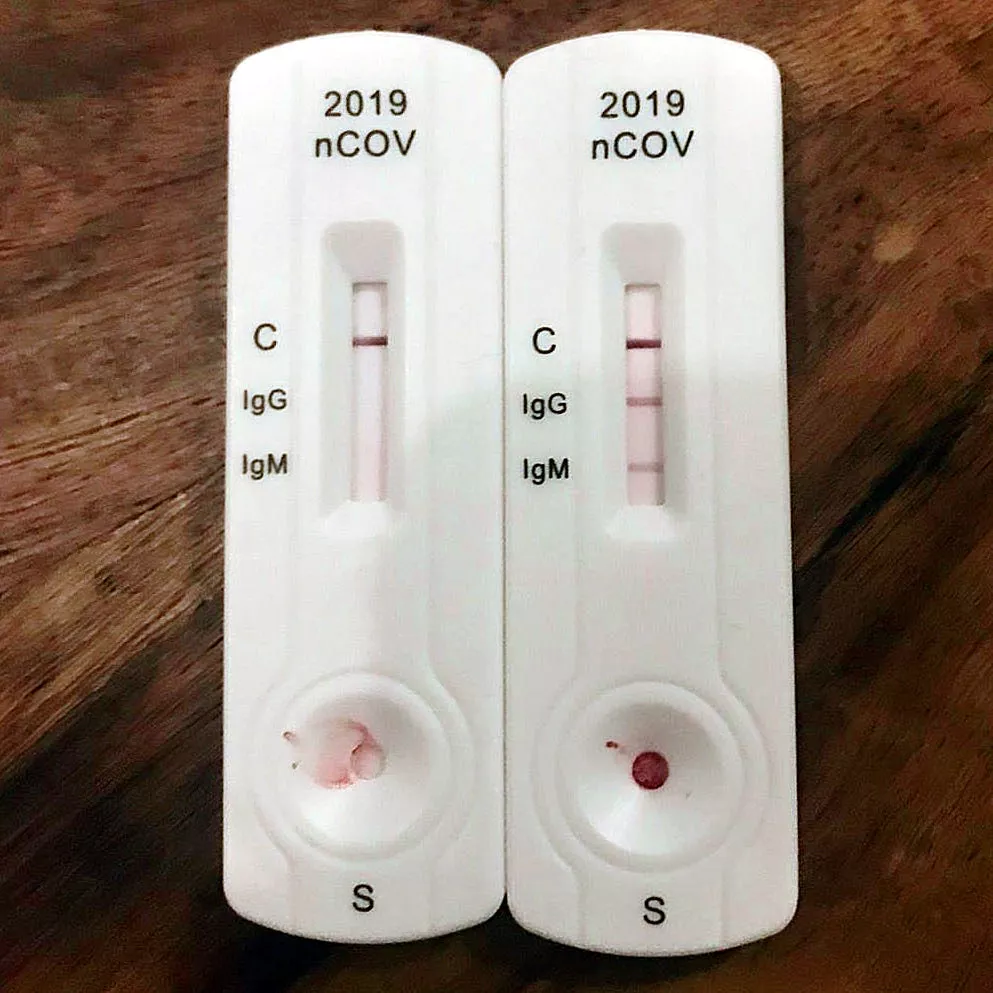 Figure source: IgG and IgM rapid diagnostic test for COVID-19 by Partynia Wikipedia - Serology This time, the sample dropped into the spiked wells may have had an effect on SARS-CoV-2 antibodies. So the binding pad should then be antigen with a marker on it - of course it's not possible to put live virus up. Generally what is used here is the antigenic protein designed to be detected, such as the N or S proteins mentioned earlier, or a recombinant virus, either of which must contain a receptor binding domain (RBD) as a target for antibody binding. On the detection line, it is the anti-IgM or anti-IgG antibody that is attached to capture the antibody protein that has bound the antigen. Finally, antibodies specific for the antigen are attached to the quality control line to capture the remaining free antigen.
Figure source: IgG and IgM rapid diagnostic test for COVID-19 by Partynia Wikipedia - Serology This time, the sample dropped into the spiked wells may have had an effect on SARS-CoV-2 antibodies. So the binding pad should then be antigen with a marker on it - of course it's not possible to put live virus up. Generally what is used here is the antigenic protein designed to be detected, such as the N or S proteins mentioned earlier, or a recombinant virus, either of which must contain a receptor binding domain (RBD) as a target for antibody binding. On the detection line, it is the anti-IgM or anti-IgG antibody that is attached to capture the antibody protein that has bound the antigen. Finally, antibodies specific for the antigen are attached to the quality control line to capture the remaining free antigen.
summary
In general, the three tests are aimed at different needs and complement each other's strengths. Nucleic acid testing is the gold standard, which directly checks the RNA of the virus and is responsible for seeing if the person is carrying the virus; antigen testing is a rapid test that checks the protein of the virus, but is not as accurate as nucleic acid testing and is more effective for highly infectious people; and antibody testing checks whether the vaccine is in effect and whether the person has recently been infected with the virus.
Recently, the New Crown epidemic has made a comeback in various parts of the world. the Omicron variant has a shorter incubation period, faster replication of the virus, and significantly greater infectivity than the previously prevalent Delta variant, although the rate of death and serious illness is significantly lower. We hope everyone stays healthy in this environment.